Invented by Robert L. Fahrner, Jayme Franklin, Paul McDonald, Thanmaya Peram, Vikram Sisodiya, Corazon Victa, Genentech Inc
Polyelectrolytes are polymers that contain charged groups, either positive or negative, along their molecular chains. These charged groups interact with proteins, leading to their precipitation and subsequent separation from the mixture. The use of polyelectrolytes in protein purification offers high selectivity, efficiency, and scalability, making it an attractive option for large-scale production.
One of the key drivers of the market is the growing demand for biopharmaceuticals. With the increasing prevalence of chronic diseases and the need for personalized medicine, the demand for high-quality proteins for therapeutic purposes is on the rise. Polyelectrolyte precipitation enables the efficient purification of proteins, ensuring their safety and efficacy for use in pharmaceutical products.
Moreover, the food processing industry is also driving the market growth. Proteins are essential ingredients in various food products, including meat substitutes, dairy alternatives, and nutritional supplements. Polyelectrolyte precipitation allows for the separation and purification of proteins from different sources, such as plant-based proteins or animal-derived proteins, ensuring the production of high-quality and safe food products.
Additionally, the advancements in biotechnology and genetic engineering have led to the production of a wide range of recombinant proteins. These proteins need to be purified from complex mixtures, and polyelectrolyte precipitation offers an efficient and cost-effective solution. The market for polyelectrolyte precipitation is expected to witness significant growth as the demand for recombinant proteins continues to increase.
Furthermore, the market is also driven by the advantages offered by polyelectrolyte precipitation over traditional purification methods. Traditional methods, such as chromatography, can be time-consuming, expensive, and may require multiple steps. Polyelectrolyte precipitation, on the other hand, is a simple and rapid technique that can be easily scaled up for large-scale production. It also offers high selectivity, allowing for the purification of specific proteins from complex mixtures.
In terms of regional analysis, North America and Europe are expected to dominate the market due to the presence of a well-established biopharmaceutical industry and increasing investments in research and development. However, the Asia Pacific region is also witnessing significant growth due to the rising demand for biopharmaceuticals and the increasing focus on healthcare infrastructure development.
In conclusion, the market for polyelectrolyte precipitation and purification of proteins is witnessing significant growth due to the increasing demand for high-quality proteins in various industries. The advantages offered by polyelectrolyte precipitation, such as high selectivity, efficiency, and scalability, make it an attractive option for protein purification. With the growing demand for biopharmaceuticals and advancements in biotechnology, the market is expected to continue its upward trajectory in the coming years.
The Genentech Inc invention works as follows
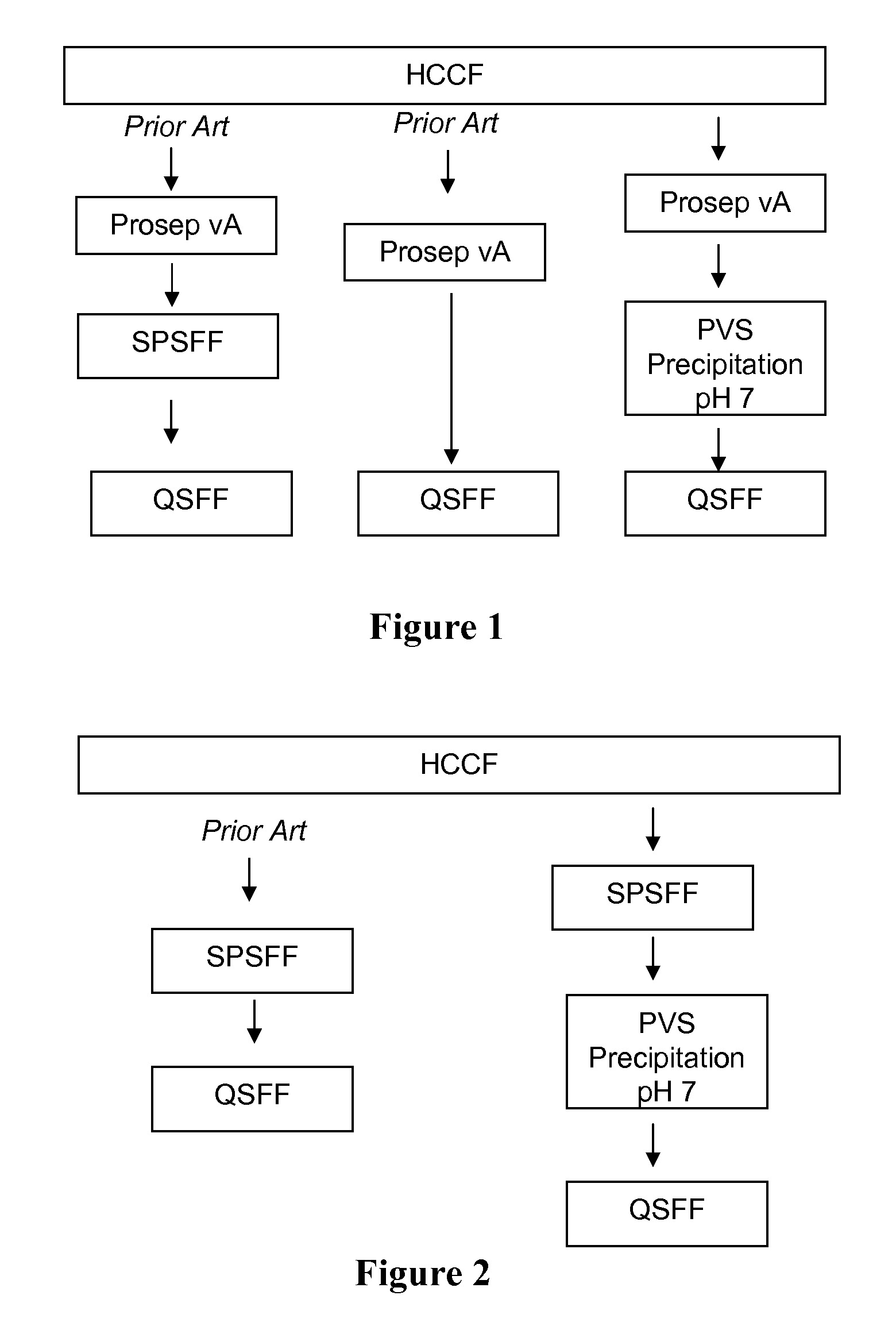
Methods are presented for isolating and purifying proteins by adding a polyelectrolyte to a cell culture fluid, such as a harvested cell culture fluid, and precipitating a protein-polyelectrolyte complex or a complex of impurities and the polyelectrolyte.
Background for Polyelectrolyte Precipitation and Purification of Proteins
The biotechnology industry is facing a growing problem: the large-scale and economic purification of protein. In general, proteins are made by cell culture using mammalian and bacterial cell cultures that have been engineered to produce a protein of interest through the insertion of the gene of the protein by a recombinant DNA plasmid. The cell lines are living organisms and must be supplied with a complex medium containing amino acids, sugars, and growth factors. This is usually done by using animal serum. The separation of the desired proteins from the compounds that are fed to the cell and the by-products from the cells to a level of purity suitable for human therapeutic use is a daunting challenge.
Advances made in cell culture and fermentation techniques have increased the concentration of target proteins within culture fluids. The increase in efficiency upstream has caused a bottleneck at the stage of cell harvesting. Cell harvesting or clarification of harvested cell culture liquid is an important downstream purification process for biotech-based product. Cell harvesting can be used when the product is inside the cells. This will reduce the volume of liquid cells that need to be processed for the extraction process. Cell harvesting can be used when the product is extracellular to separate it from the cells, cellular debris and the cells themselves. For example, an extracellular antibody was isolated from mammalian culture by Anthony S. Lubiniecki (Ed.). (1990) Large-Scale Mammalian Cell Culture Technology, Marcel Dekker; Hansjoerg Hauser, Roland Wagner, Eds. “Mammalian Cell Biotechnology for Protein Production (Walter Gruyter Publishing)
Procedures to purify proteins from cell debris depend initially on the site of protein expression.” Some proteins are secreted from the cells directly into the growth medium, while others are produced intracellularly. The first step in a purification procedure for the latter proteins is lysis of cells. This can be achieved by various methods including mechanical shears, osmotic treatments, or enzyme treatments. This disruption allows the contents of the cells to be released into the homogenate. It also produces small subcellular fragments, which are hard to remove because they’re so small. They are usually removed using differential centrifugation, or by filtering. Directly secreted protein problems are also present, but on a smaller level, due to the death of cells during the production of proteins and the release of host cell proteins.
During the purification process of therapeutic antibodies impurities such as host cell proteins and product variants must be removed. Small molecules, contaminants related to the processing, endotoxins, viral particles, and host cell DNA are also present (Fahrner R. L. Genet. Eng. Rev. 18:301-327). Purification techniques must be scalable, cost-effective and reliable. They also need to meet the strict purity requirements for the final product. The current purification methods involve multiple chromatographic separates using orthogonal modes. Precipitation (U.S. No. No. Column chromatography is a reliable and effective method, but it has a low throughput (kg/h). “As monoclonal antibody production becomes more popular, more efficient processes-scale production will be required.
Chromatography uses the chemical and physical characteristics of proteins to achieve high levels of purification.” These physical and chemical properties include, for example, size, isoelectric points, charge distributions, hydrophobic site affinity, and hydrophobic sites (Janson J. C., and L. Ryden, eds.). (1989) Protein Purification : Principles, high resolution methods and applications. VCH Publishers, Inc., New York). Separation modes in chromatography are ion exchange, chromatofocusing (size exclusion), gel filtration, reverse phase and affinity chromatography. The ion-exchange chromatography, including anion exchange and cation exchange chromatography, separates analytes such as proteins. Proteins are separated by the differences in their surface charges. IEX is a tool that can be used to separate expressed proteins from cellular debris or other impurities. IEX has become one of the most widely used purification techniques, especially for proteins, peptides and nucleic acid. It offers high-resolution group separations and high loading capacities. The technique can separate molecular species with only minor differences in charge properties. For example, two proteins that differ by just one charged amino acid. This makes IEX a good choice for intermediate purification, polishing or capture steps in a purification procedure. The technique can be used to purify kilograms of products.
Chromatography is reliable, but the capacity and throughput of large-scale applications can be problematic.” The conventional column chromatography is reliable and effective, but has a low throughput (kg/h). The use of recombinant protein is increasing, and therefore more efficient production at the process scale will be required. The capacity of the resin used in chromatography is usually what limits the throughput. “With increased protein loading to the column the resolution of the protein is often decreased.
Polyelectrolytes can form complexes that are soluble with proteins (Dellacherie E. (1991), Am. Chem. Soc., Div. Polym. Chem. Prepr. 32(1):602), amorphous precipitates (Mattiasson et al (1998) Polym. Plast. Technol. Eng. 37(3):303-308; Clark et al (1987) Biotech. Progress 3(4):241; Fisher et al (1988) Biotechnol. Bioeng. 32:777; Shieh et al (1991) Am. Chem. Soc., Div. Polym. Chem. Prepr. 32(1)606; Sternberg et al (1974) Biochimica et Biophysica Acta 342:195-206; WO 2004/014942), or coacervates (Wang et al (1996) Biotechnol. Prog. 12:356-362; Veis, A. (1991) Am. Chem. Soc. Div. Polym. Chem. Prepr. 32(1) 596). In the presence of polymethacrylic acids (antigen-polycation), papain is used to break down monoclonal antibody into Fab fragments. (Dainiak and colleagues, 2000 Analytical Biochem. 277:58-66).
Protein-polyelectrolyte complexes coacervate, i.e. Separate into two distinct liquid phase where the coacervate contains the majority of the complex, and the other phase represents the equilibrium phase. Complex Coacervation – Microcapsule Formation Macromolecular Complexes, Dubin, P. L. et al. Eds. (1994) Springer-Verlag, Berlin; Dubin et al (1994) Sep. Purif. Methods 23:1-16). The polyelectrolyte-coacervation process is complex and not suitable for all proteins. The intermolecular associations in protein-polyelectrolyte complexes are due to electrostatic interactions, hydrogen bonds and hydrophobic forces (Cooper et al (2005) Current Opinion in Colloid & Interface Science 10:52-78; Mattison et al (1999) Macromol. Symp. 140:53-76). While it is known that addition of a polyelectrolyte to a protein solution can lead to the formation of protein-polyelectrolyte complexes and larger clusters and eventually to a coacervate and/or precipitation, the reverse process may appear upon further addition of polyelectrolyte whereby redissolution of protein occurs, defeating the attempt at protein isolation or purification (Carlsson et al (2003) J. Am. Chem. Soc. 125:3140-3149). The use of polyelectrolytes to precipitate proteins may be a more cost-effective method than chromatography. This technique could be used to use the functional chemistry behind chromatographic methods to achieve similar levels of purification of proteins in solution. The precipitation step’s throughput would not be restricted by the capacity or a particular chromatography material.
The invention relates to methods for the isolation and purification proteins derived from cells culture fluids.
One aspect of the invention is purification of proteins by precipitation with polyelectrolytes, such as polyanion polyelectrolytes.
The invention provides a process for purifying proteins by precipitating impurities in cell culture fluids with a polycation electrolyte. This precipitation step can be followed by cation-exchange chromatography or anion-exchange chromatography and other precipitation stages.
The invention includes a process that does not require affinity for purifying an antibody.
One aspect is a purification method for antibodies that comprises:
(a), adjusting the acidity of salt concentration in a mixture that contains an antibody, where the antibody is derived by a harvested culture fluid;
(b) adding a negatively charged polyelectrolyte whereby a protein-polyelectrolyte precipitate is formed;
(c) separating the protein-polyelectrolyte precipitate from impurities selected from protein aggregates, protein fragments, host cell proteins, insulin, gentamicin, DNA, and leached protein A;
(d) isolating the protein-polyelectrolyte precipitate; and
(e) resuspending the protein-polyelectrolyte precipitate in an aqueous solution.
Another aspect is a purification method for antibodies that comprises:
(a), adjusting the acidity of salt concentration in a mixture that contains an antibody, where the antibody is derived by a cell-culture fluid;
Click here to view the patent on Google Patents.