Invented by Svetlana Doronina, Robert Lyon, Peter Senter, Seagen Inc
ADCs are designed to selectively deliver cytotoxic drugs to cancer cells while sparing healthy cells, minimizing side effects commonly associated with chemotherapy. This targeted approach improves the efficacy of treatment and enhances patient outcomes. However, traditional ADCs face challenges related to stability, off-target toxicity, and limited payload capacity. Hydrophilic ADCs address these limitations by incorporating hydrophilic linkers, which improve stability and reduce off-target toxicity.
The market for hydrophilic ADCs is driven by several factors. Firstly, the rising incidence of cancer worldwide is fueling the demand for novel and effective treatment options. According to the World Health Organization, cancer is the second leading cause of death globally, with approximately 9.6 million deaths in 2018. Hydrophilic ADCs offer a promising solution for targeted cancer therapy, driving their adoption in the market.
Moreover, advancements in antibody engineering and conjugation technologies have facilitated the development of hydrophilic ADCs with improved stability, specificity, and drug release kinetics. These technological advancements have attracted significant investment from pharmaceutical companies, leading to a robust pipeline of hydrophilic ADC candidates.
Furthermore, the increasing focus on personalized medicine and precision oncology has created a favorable environment for the growth of hydrophilic ADCs. These therapeutics can be tailored to target specific cancer types or even individual patient populations, allowing for more personalized treatment approaches. This customization potential enhances the therapeutic efficacy and reduces the chances of resistance development.
The market for hydrophilic ADCs is also supported by favorable regulatory policies and incentives. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA), have implemented expedited review pathways for breakthrough therapies, including ADCs. This regulatory support encourages pharmaceutical companies to invest in the development of hydrophilic ADCs, driving market growth.
However, challenges remain in the market for hydrophilic ADCs. Manufacturing complexities, high costs, and the need for specialized infrastructure pose barriers to widespread adoption. Additionally, the competitive landscape is becoming increasingly crowded, with several companies vying for market share. This intensifying competition necessitates continuous innovation and differentiation to succeed in the market.
In conclusion, the market for hydrophilic ADCs is witnessing significant growth due to their potential to revolutionize targeted cancer therapy. The rising incidence of cancer, technological advancements, personalized medicine trends, and regulatory support are driving the adoption of hydrophilic ADCs. However, challenges related to manufacturing complexities and competition need to be addressed to fully realize the market’s potential. As pharmaceutical companies continue to invest in research and development, hydrophilic ADCs hold immense promise for improving patient outcomes and transforming the landscape of cancer treatment.
The Seagen Inc invention works as follows
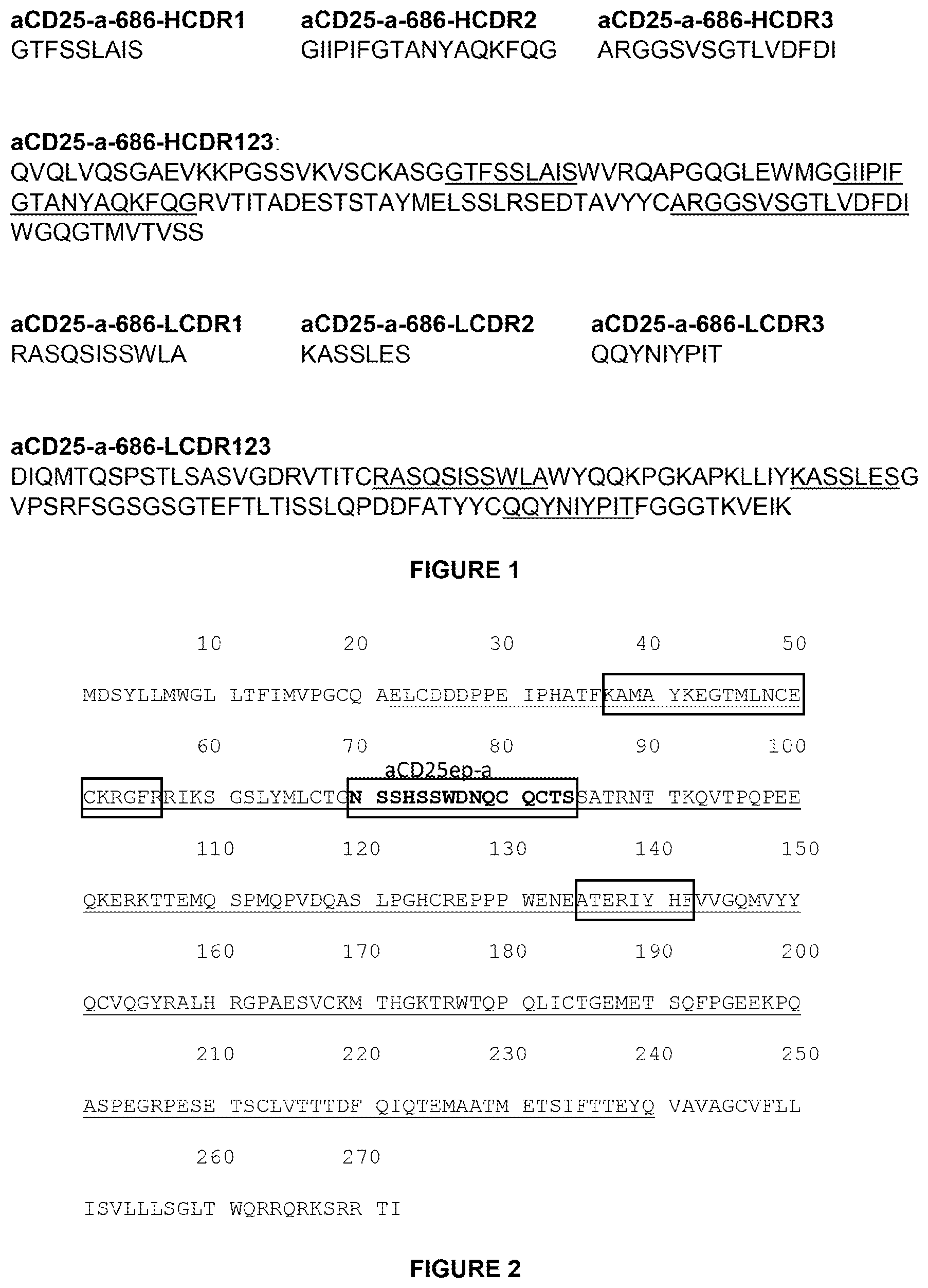
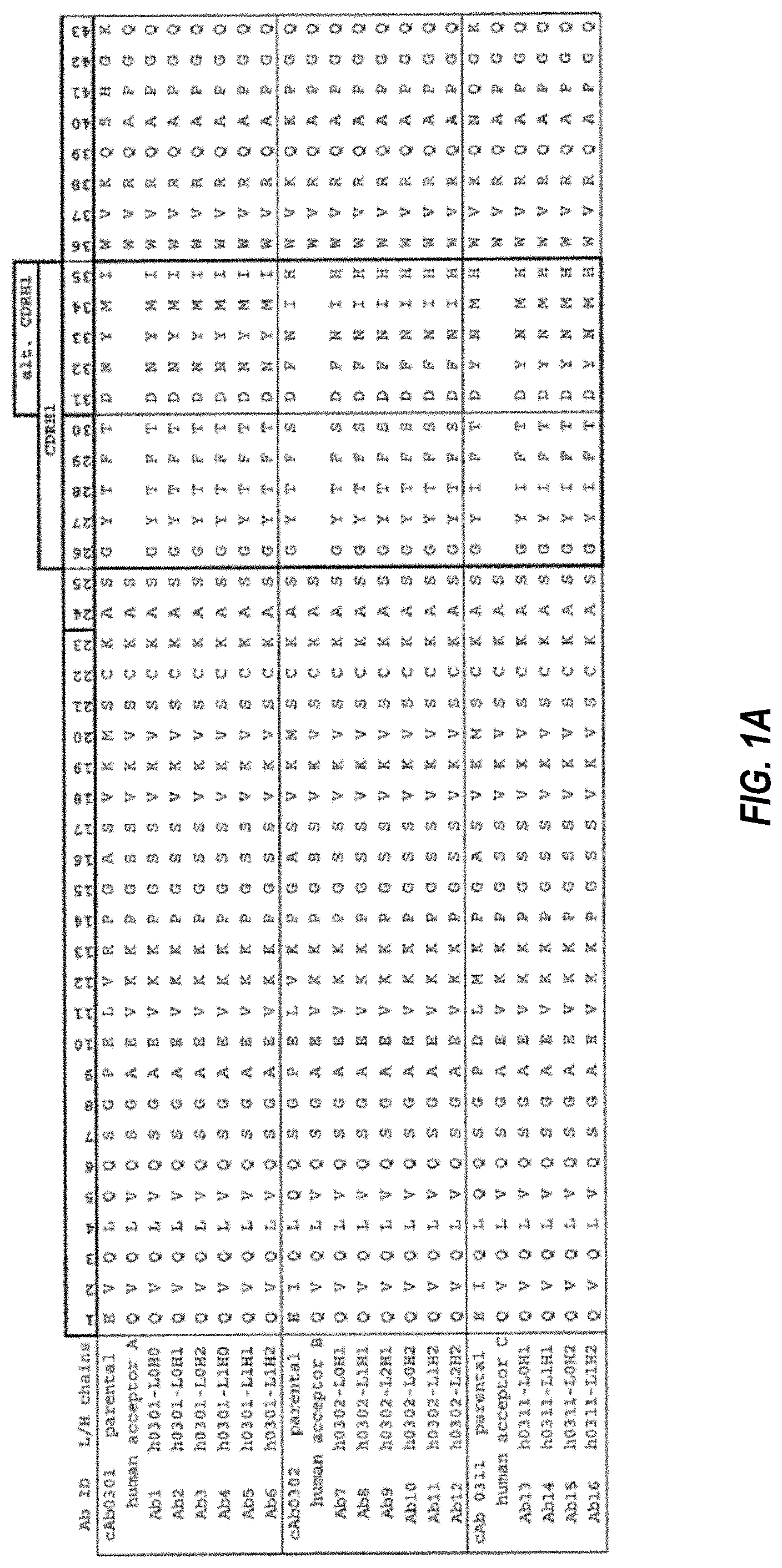
The following are described: “Hydrophilic linkers, Drug-Linker Compounds, Drug Ligand Conjugate Compounds and Ligand-Linkers as well as methods for making and using them.
Background for Hydrophilic antibody-drug conjugates
General
Ligand-Linker Drug Conjugates
Activity Assays
Drug-Linker Compounds
Linkers
Linker-Ligand Conjugates
Treatment of Diseases
Treatment of Cancer
Compositions & Methods of Administration
Assembly of the Ligand Drug Conjugates
EXAMPLES
General
Abbreviations
Example 1 – General Procedures for Syntheses
Synthesis of Maleimido-Dpr(Boc)-OH
Example 2?Drug Linkers
Example 3?Antibody Drug Conjugates
In Vitro Activity Tests: Example 4
Example 5?Pharmacokinetics Studies
Example 6?In Vivo Therapy Experiments
Click here to view the patent on Google Patents.