Invented by Mark White, Sandeep Kumar, Christopher Chan, Spencer Liang, Lance Stapleton, Andrew W. Drake, Yosi Gozlan, Ilan Vaknin, Shirley Sameah-Greenwald, Liat Dassa, Zohar Tiran, Gad S. Cojocaru, Leonard Presta, Richard Theolis, Compugen Ltd, Compugen USA Inc
In recent years, the field of immunotherapy has revolutionized cancer treatment by harnessing the power of the immune system to fight against cancer cells. One promising avenue in this field is the development of antibodies that target immune checkpoints, molecules that regulate the immune response. One such checkpoint is PVRIG, and the market for anti-PVRIG antibodies is gaining significant attention.
PVRIG, also known as Poliovirus Receptor-Related Immunoglobulin Domain-Containing Protein, is a cell surface receptor that plays a crucial role in regulating immune responses. It is expressed on various immune cells, including T cells and natural killer (NK) cells. PVRIG interacts with its ligand, PVRL2, which is expressed on tumor cells, leading to the suppression of immune responses against cancer.
Anti-PVRIG antibodies work by blocking the interaction between PVRIG and PVRL2, thereby preventing the immune suppression caused by this interaction. By inhibiting PVRIG, these antibodies can enhance the anti-tumor immune response, allowing the immune system to better recognize and eliminate cancer cells.
The market for anti-PVRIG antibodies is driven by the increasing prevalence of cancer worldwide and the need for more effective treatment options. According to the World Health Organization (WHO), cancer is one of the leading causes of death globally, with approximately 10 million deaths in 2020 alone. Conventional cancer treatments, such as chemotherapy and radiation therapy, often have limited efficacy and can cause significant side effects. Therefore, there is a growing demand for novel immunotherapies like anti-PVRIG antibodies that can provide more targeted and less toxic treatment options.
Several pharmaceutical companies and biotechnology firms are actively involved in the development of anti-PVRIG antibodies. These companies are conducting preclinical and clinical trials to evaluate the safety and efficacy of these antibodies in various cancer types. The results from early-stage clinical trials have shown promising outcomes, with some patients experiencing tumor regression or stabilization.
The market for anti-PVRIG antibodies is expected to witness substantial growth in the coming years. Factors such as increasing investments in research and development, favorable regulatory environment, and rising collaborations between pharmaceutical companies and academic institutions are driving this growth. Moreover, the potential for combination therapies, where anti-PVRIG antibodies are used in conjunction with other immunotherapies or standard treatments, further expands the market opportunities.
However, challenges remain in the development and commercialization of anti-PVRIG antibodies. One major hurdle is the identification of biomarkers that can predict patient response to these antibodies. Personalized medicine approaches, where patients are selected based on specific biomarkers, can enhance treatment outcomes and reduce unnecessary costs. Additionally, the high cost of antibody production and the need for large-scale manufacturing facilities pose challenges in terms of affordability and accessibility.
In conclusion, the market for anti-PVRIG antibodies is a rapidly growing field within the immunotherapy landscape. These antibodies hold great promise in enhancing the immune response against cancer cells and improving patient outcomes. As research and development efforts continue, it is expected that anti-PVRIG antibodies will become an integral part of the cancer treatment armamentarium, providing new hope for patients battling this devastating disease.
The Compugen Ltd, Compugen USA Inc invention works as follows
The present invention relates to anti-PVRIG antibody and methods for using them.
Background for Anti-PVRIG antibodies, and their use
Costimulatory molecules pairs are usually composed of receptors on T-cells and ligands on APCs. The prototype costimulatory molecule receptor/ligand pairs are CD40/CD40L and B7/CD28. The B7 family is composed of structurally related cell-surface proteins ligands that can provide either stimulatory or inhibiting input to an immunity response. The B7 family is structurally related. Each member has at least one variable immunoglobulin or constant immunoglobulin in the extracellular domain.
Positive and negative costimulatory signal play critical roles in the control of cell-mediated immunity. Molecules that mediate those signals have been proven to be effective immunomodulation targets. This knowledge has led to the development of several therapeutic approaches that target costimulatory molecule. These have proven useful in treating cancer, autoimmune and inflammatory disease, and rejection of allogenic organ transplants. By negative costimulation or coinhibition in subjects with these pathological diseases.
Manipulation by B7 ligands of signals has shown promise in the treatment for autoimmunity and inflammatory diseases as well as transplant rejection. Monoclonal antibody blocking is one of the therapeutic strategies. It can be done by using monoclonal antigens to the receptor or ligand of a pair of costimulatory ligands, or soluble fusion protein composed of a costimulatory ligand that may bind to and block the appropriate ligand. Induction of co-inhibition is another approach that uses a soluble fusion of an inhibitory receptor. These approaches depend, at least in part, on the eventual elimination of auto- and allo-reactive cells (which are the ones responsible for the pathogenic process in autoimmune disease or transplantation respectively). This is presumably because, in the absence costimulation (which induces cells survival genes), T cells are highly susceptible to apoptosis. Novel agents that modulate costimulatory signaling without compromising the ability of the immune system to defend itself against pathogens are therefore highly beneficial for treating and preventing such pathological conditions.
Costimulatory pathway play an important part in tumor development. It is interesting to note that tumors can evade immune destruction through the inhibition of costimulatory factors such as B7-CD28, TNF, and regulatory T cells which inhibit anti-tumor T-cell responses. Cancer. Biol. 16:73-79; Greenwald, et al. The B7 family revisited, Ann. Rev. Immunol. Watts (2005). “TNF/TNFR family members in co-stimulation T cell responses? Ann. Rev. Immunol. 23:23-68. Sadum et al. (2007), “Immune signatures of murine and human cancers reveal unique mechanisms of tumor escape and new targets for cancer immunotherapy”, Clin. Canc. Res. 13(13): 4016-4025). These tumor-expressed co-stimulatory molecule have been identified as attractive cancer biomarkers, and they may also serve as tumor-associated antibodies (TAAs). Costimulatory pathways are also immunologic checkpoints which attenuate immune responses triggered by T cells, both in the initiation phase and at the effector stage of tumor metastases. It is becoming increasingly clear that immunologic checkpoints can be a barrier to the ability of engineered cancer vaccines to induce therapeutic antitumor responses. Costimulatory molecules are useful as adjuvants in active (vaccination), passive (antibody-mediated), and cancer immunotherapy. They can be used to stimulate the immune system and thwart immunity tolerance.
The invention is intended to provide PVRIG immune modulatory antibodies.
The invention aims to activate a subset (or subsets) of CTLs in a patient by administering anti-PVRIG antibodies to the patient.
The invention also aims to activate a subset NK-cells of a patient by administering anti-PVRIG antibodies to the patient.
It is a further object of this invention to provide means of activating the? ? T cells of a?patient comprising the administration of an anti-PVRIG antigen to the patient. ? “The T cells are activated.
The invention also provides methods for activating Th1-cells in a patient by administering anti-PVRIG antibodies to the patient. A subset of Th1-cells of the patient is activated.
It is also an object of the invention, to provide methods to inhibit the interaction between PVRIG and PVLR2 when a patient has a condition that is associated with this interaction. This includes administering anti-PVRIG antibodies to the patient.
The invention also provides methods for treating cancer, which include administering anti-PVRIG antibodies to the patient.
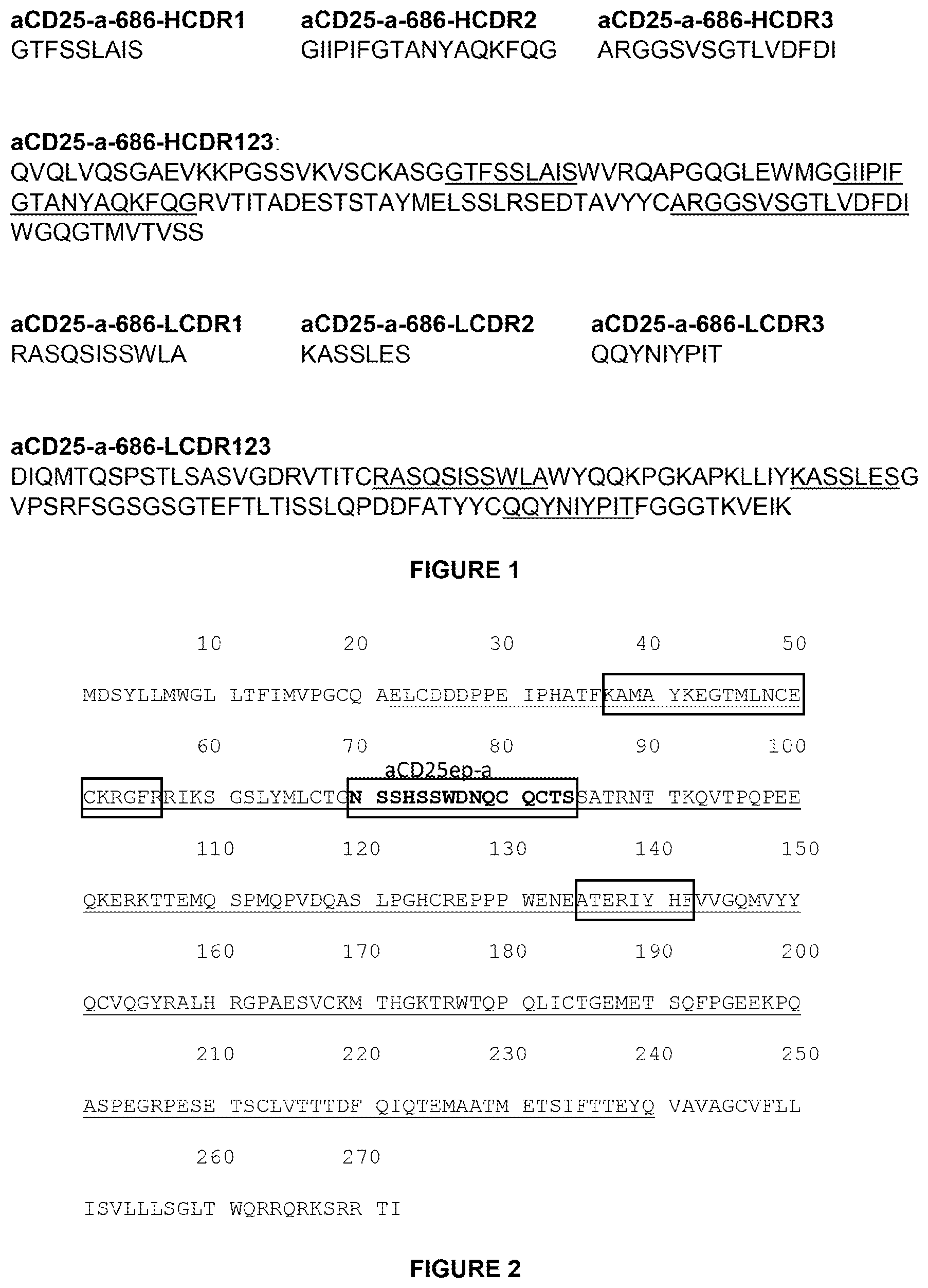
It is also an object of the invention that the anti-PVRIG antibodies are derived from an antibody chosen from CPA.7.001 to CPA.7.006 and from CPA.7.008 to CPA.7.009.
It is also an object of the invention that the anti-PVRIG antibodies compete for binding with antibodies containing the sequences of vhCDR1, CPA.7.002, vhCDR3, CPA.7.008 CPA.7.009 CPA.7.010 CPA.7.011 CPA.7.012 CPA.7.013 CPA.7.014 CPA.7.015 CPA.7.017 CPA.7.018 CPA.7.019 CPA.7.021 CPA.7.022 CPA.
It is also an object of the invention that the anti-PVRIG antibodies are selected from CPA.7.001 to CPA.7.024.
It is also an object of the invention that the anti-PVRIG antibodies compete for binding with an other antibody from the group of CPA.7.001 to CPA.7.004 and CPA.7.006 to CPA.7.009 CPA.7.010 CPA.7.011 CPA.7.012 CPA.7.013 CPA.7.014 CPA.7.015 CPA.7.017 CPA.7.018 CPA.7.019 CPA.7.021 CPA.7.022 CPA.7.023 CPA.
The invention also provides methods where the anti-PVRIG antibodies are composed of the vhCDR1, CDR2, CDR3, and vlCDR1 sequences of an antibody selected from CHA.7.502 CHA.7.503 CHA.7.506 CHA.7.508 CHA.7.509 CPA.7.010 CPA.7.011 CPA.7.012
The invention also provides methods whereby the anti-PVRIG antibodies compete for binding with other antibodies selected from a group that includes CHA.7.502 CHA.7.503 CHA.7.506 CHA.7.508 CHA.7.009 CPA.7.010 CPA.7.011
The invention also provides methods for diagnosing cancer, which include a) contacting tissue from a cancer patient with an anti PVRIG antibody and b), determining whether there is an overexpression of PVRIG within the tissue to indicate the presence or absence of cancer. The anti-PVRIG antibodies can be described as outlined in the above and herein.
The invention also provides antigen-binding domains that are antibodies against PVRIG, and which contain the vhCDR1, CPA.7.002, vhCDR2, CPA.7.03, vlCDR1, CPA.7.02, vlCDR2, and vlCDR3 from an antigen selected from CPA.7.001 to CPA.7.004. CPA.7.008 CPA.7.009 CPA.7.010 CPA.7.011 CPA.7.012 C
Click here to view the patent on Google Patents.